Track Clinical Trial Visits
Planfix turns patient visits, trial logs, and sponsor SLAs into one controlled system so your site stays inspection ready and trusted by sponsors.
Planfix turns patient visits, trial logs, and sponsor SLAs into one controlled system so your site stays inspection ready and trusted by sponsors.
Trial operations depend on three critical areas. You need clear visit and SLA rules, a realistic schedule for subjects and staff, and complete documentation for every visit. Planfix brings these pieces into one controllable workflow.
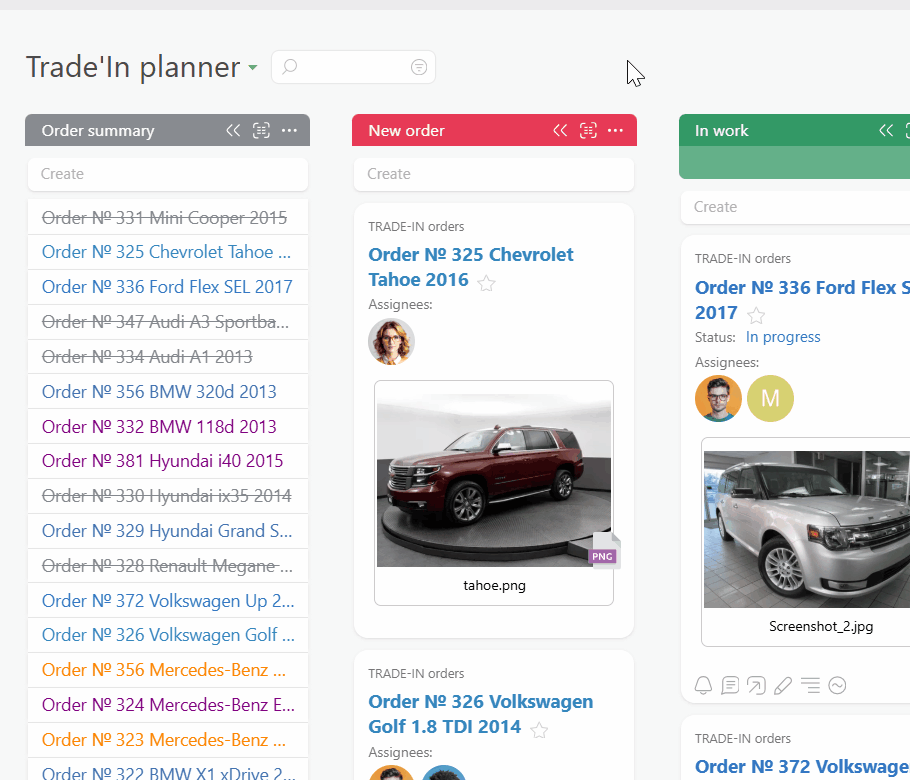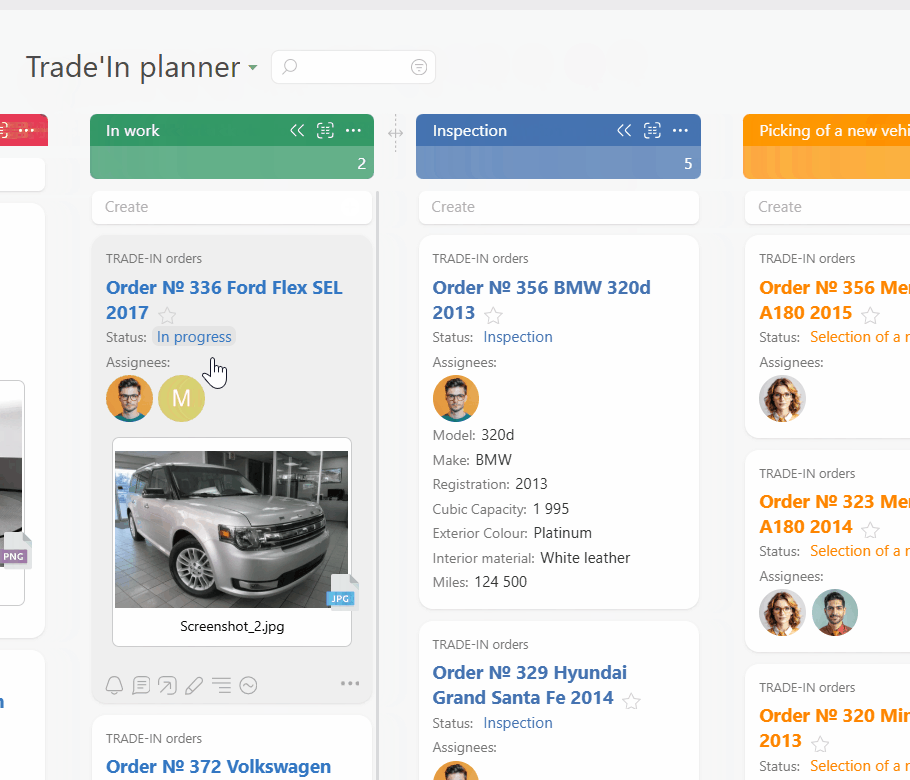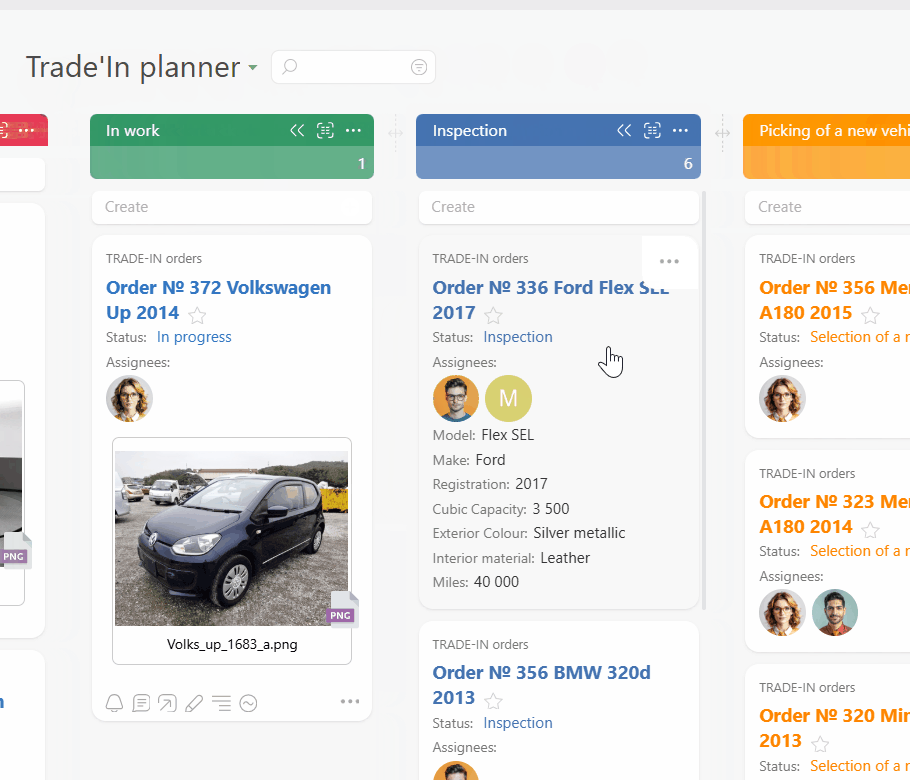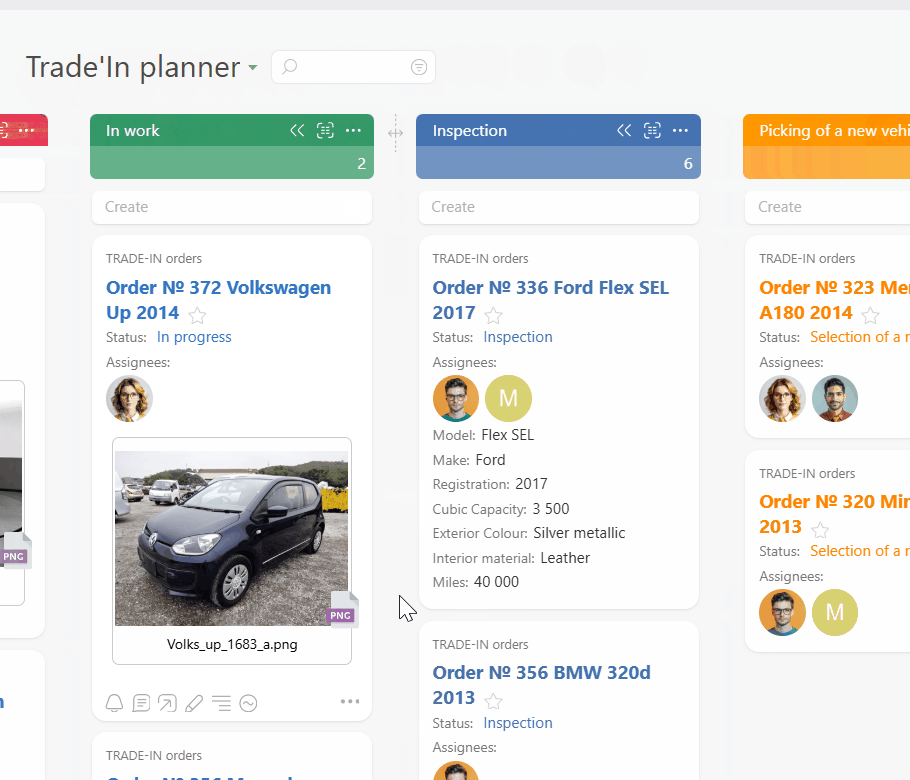
Start by centralizing sponsor and monitor requests in Planfix. Every email becomes a task so no query hides in a personal inbox. Auto triage routes work by study, subject, and SLA so the right person sees it in time.
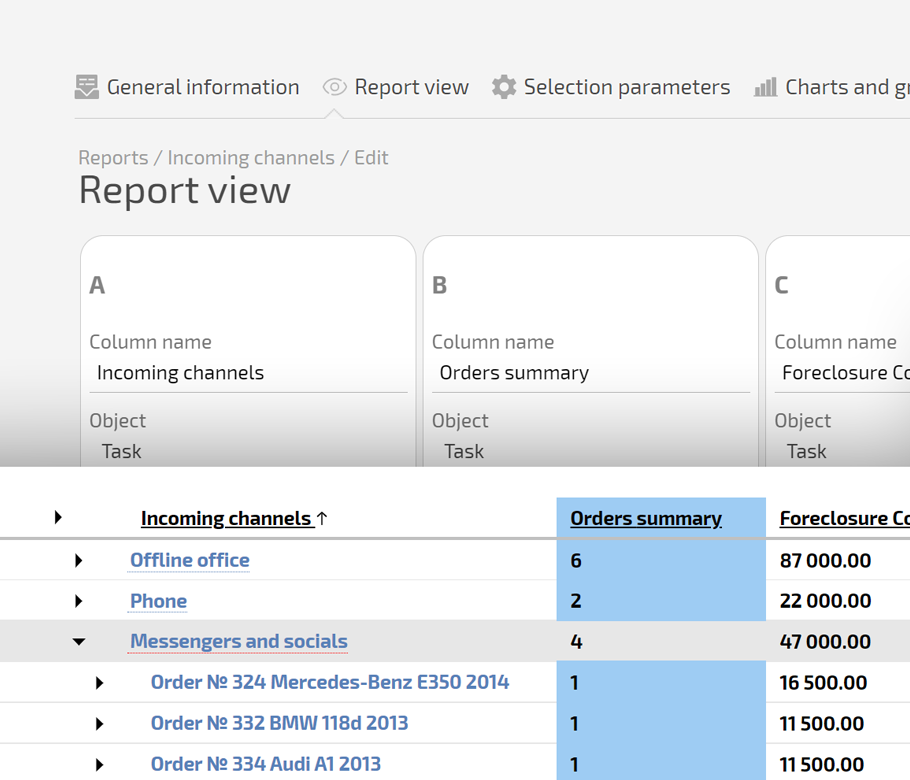
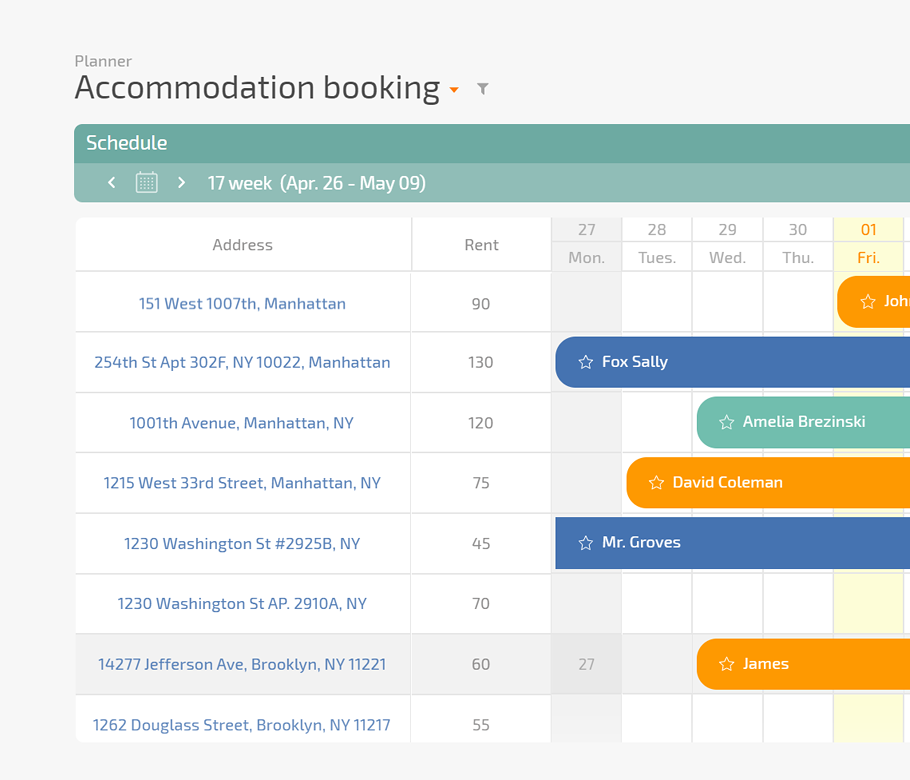
Use a Planner board where each card is a subject visit with SLA timers, status, and next steps in one line. Add dashboards where Data Tags feed visit logs, deviations, and query age into clear charts for each sponsor.
You track visits and logs in scattered spreadsheets and long email threads so the team never shares one reliable picture.
You learn about missed SLA deadlines when sponsors escalate and coordinators scramble to rebuild the story from many tools.
You fight with scattered logs, portals, and binders during monitoring visits and worry that overlooked details will turn into findings.
You spend late evenings catching up on data entry and old queries because work lives in disconnected tools and private mailboxes.
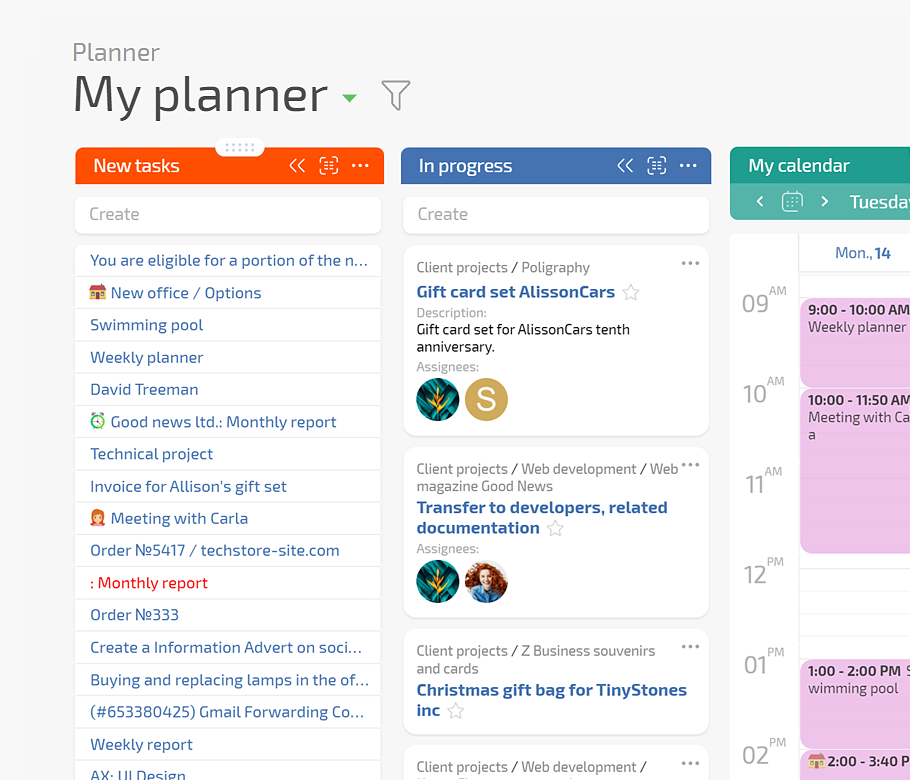
Turn protocol rules into templates and checklists so every visit task carries the right steps and documents. Use Planner with conditional lists and work calendars so subjects move smoothly from scheduled to completed without overloading staff on busy days.

Use Data Tags for dosing, temperature, and time logs so every entry is structured and reportable. Attach source files to each visit task so monitors see a complete history. Reports highlight deviations and late data entry before they become findings.
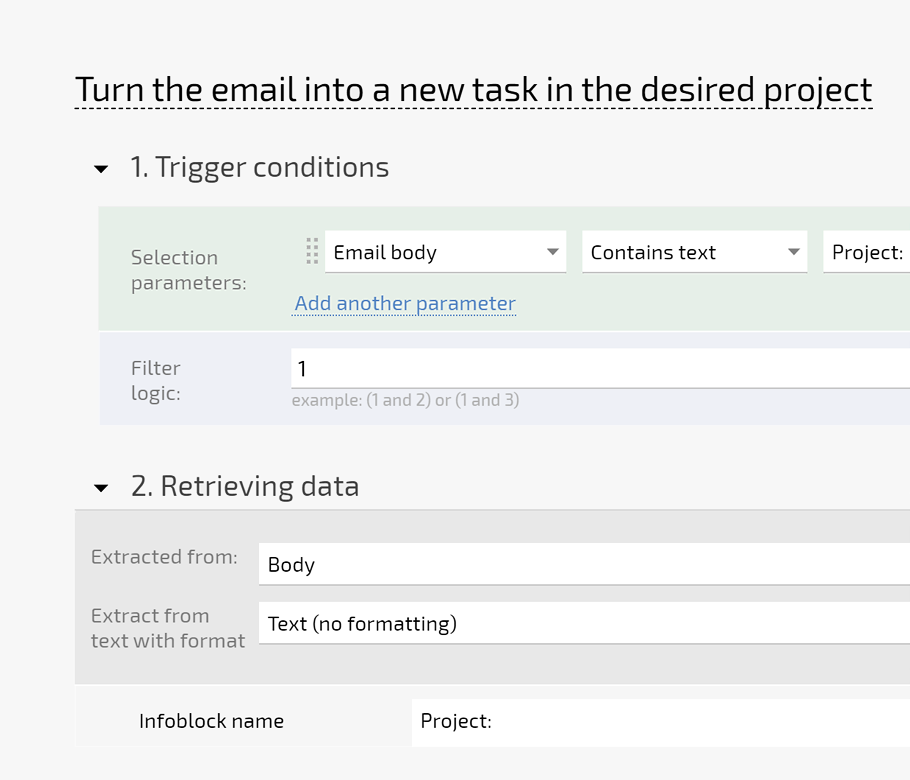
Convert sponsor emails into tasks with email to ticket so nothing is lost in personal inboxes. Auto triage, SLA fields, and Planner views keep urgent queries on top so coordinators respond faster and with less stress.

Your site runs clinical trials with calm control. Visits start on time, logs stay complete, and sponsor SLAs stay visible. New staff learn your process from Planfix instead of scattered notes so audits feel like planned reviews.
Connect email, calendars, telephony, and storage so clinical work, communication, and documents stay linked in one workflow.
See all integrations