Manage Biotech R&D Requests
Planfix runs your R&D requests, experiments, and incidents in one auditable system so you protect data integrity, move faster, and stay inspection ready.
Planfix runs your R&D requests, experiments, and incidents in one auditable system so you protect data integrity, move faster, and stay inspection ready.
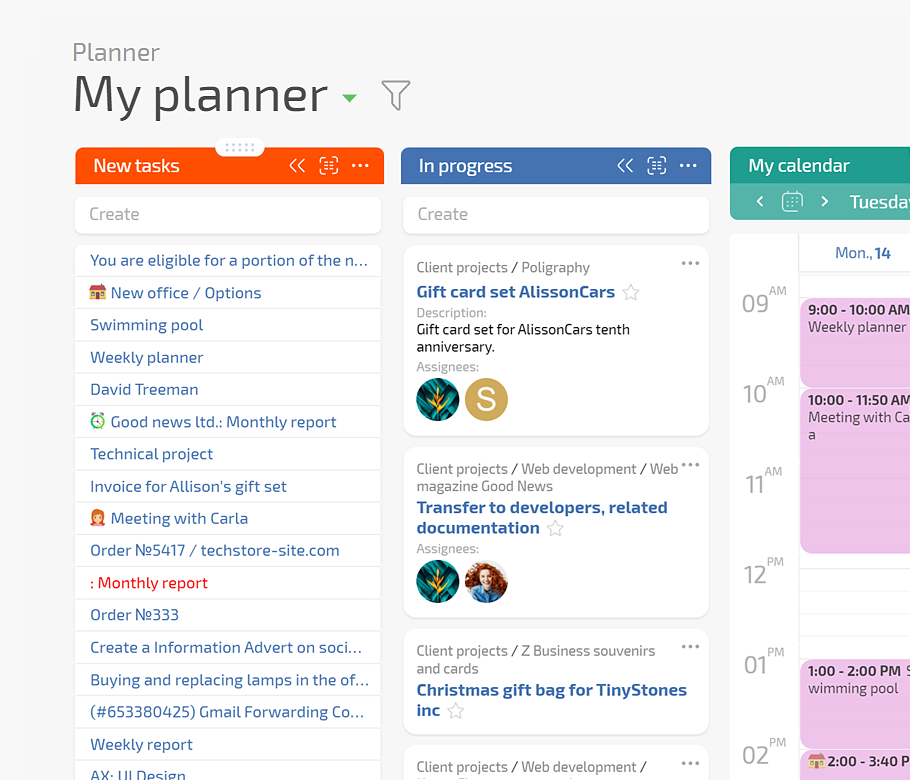
Planfix keeps every request, experiment, deviation, and decision in one configurable workspace. Planner boards, AI Dataminer, Data tags, and Project Totals replace scattered files and inboxes so your team sees live work and impact in a few clicks.
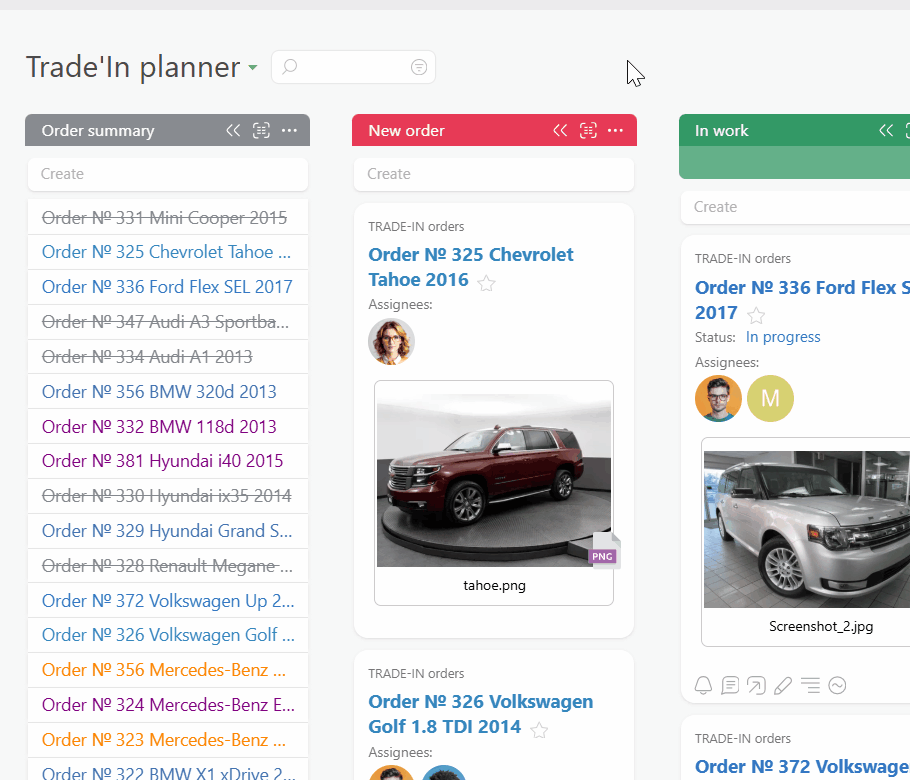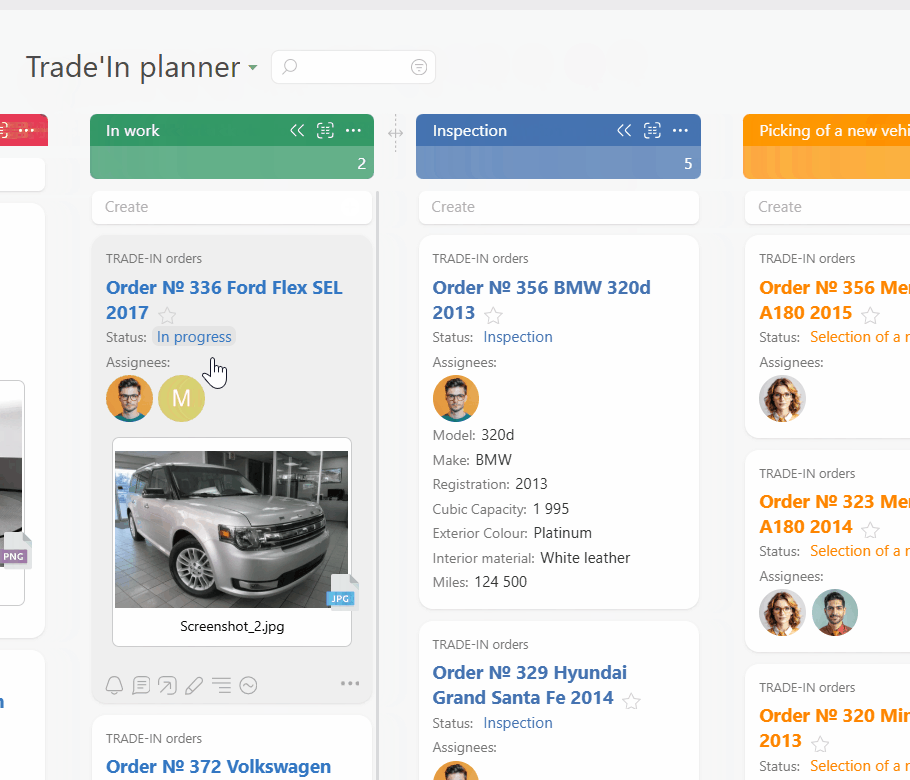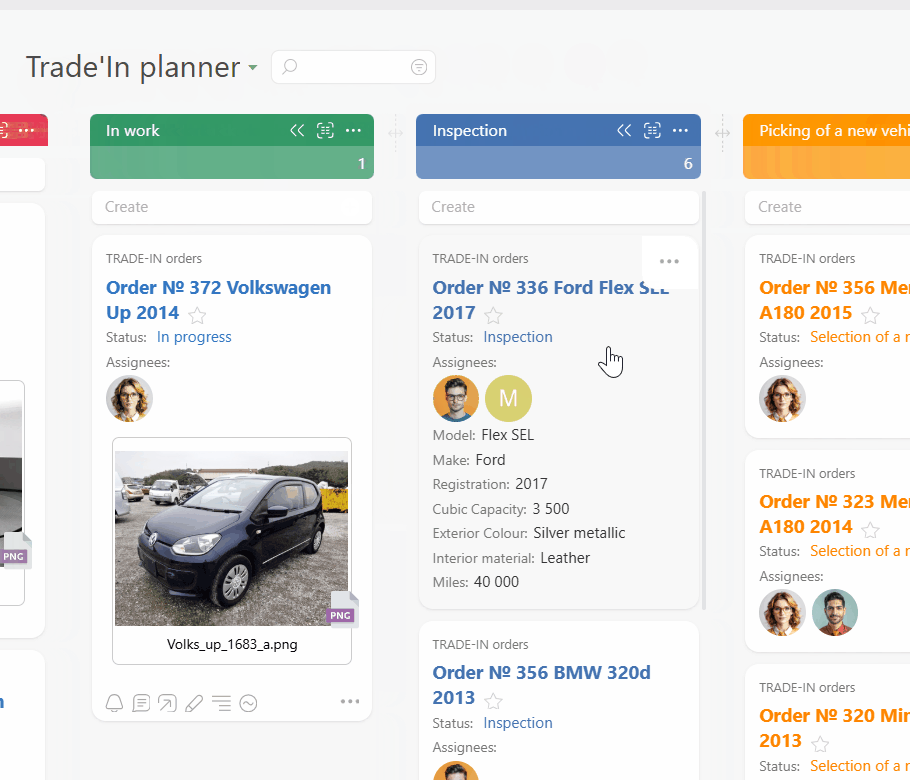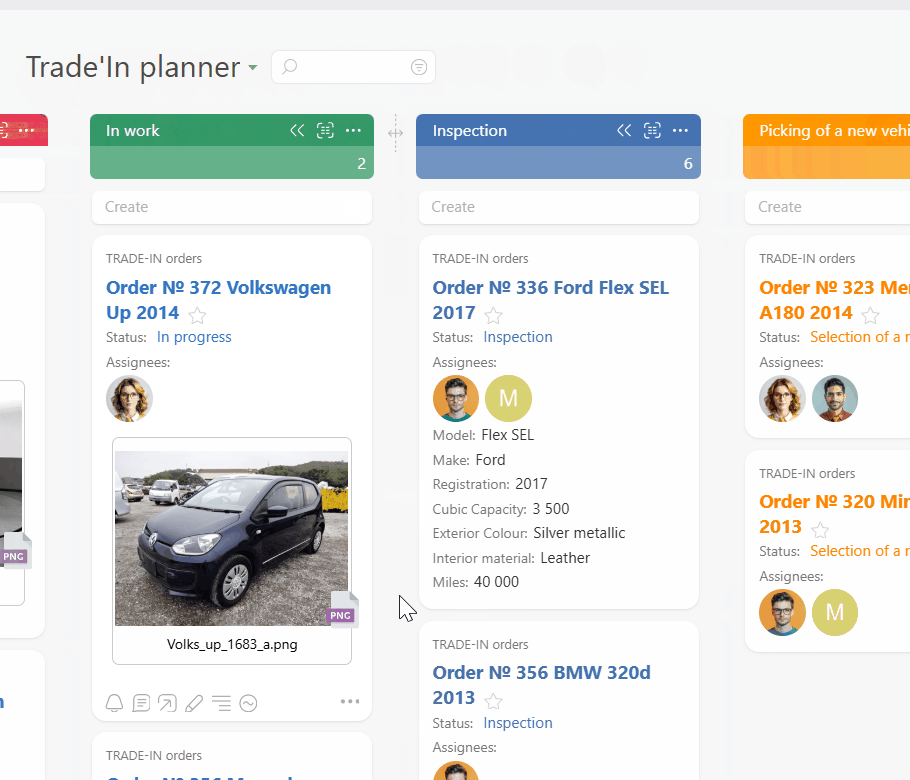
Route every request and incident into a single Planner board. Email to ticket and channel to task feed work from inboxes and chat. Conditional lists keep intake, active work, and incidents organized so you always see everything in flight.
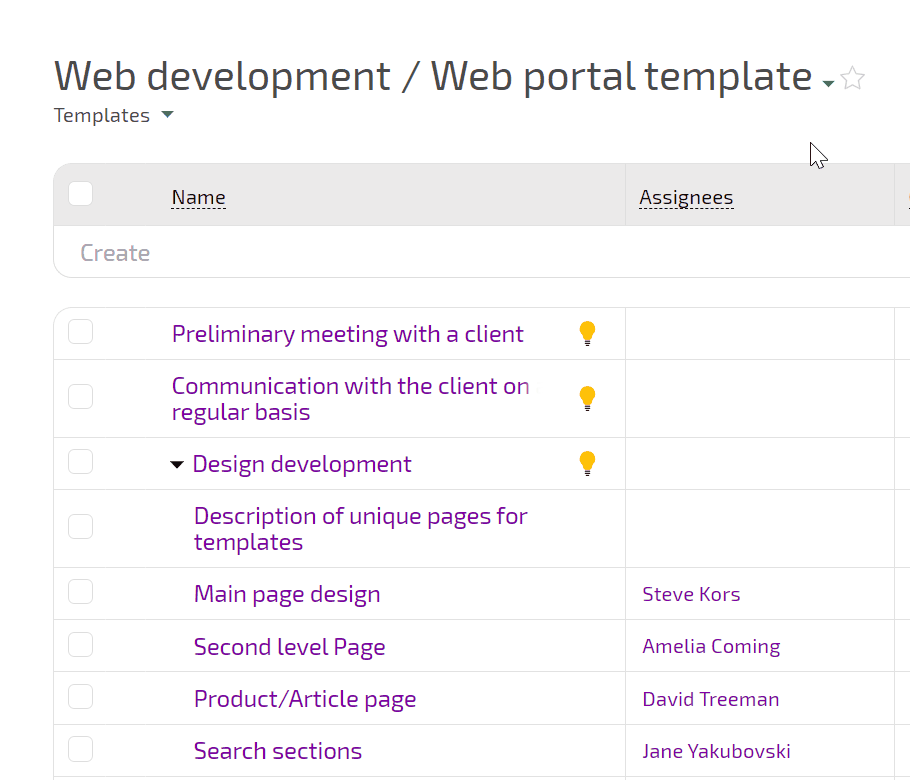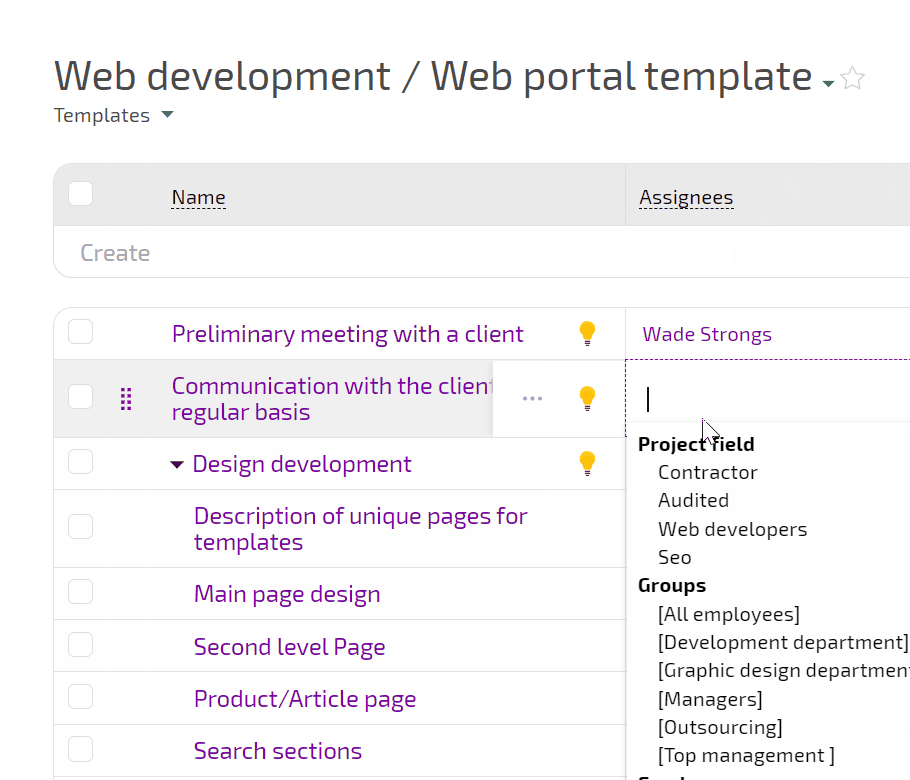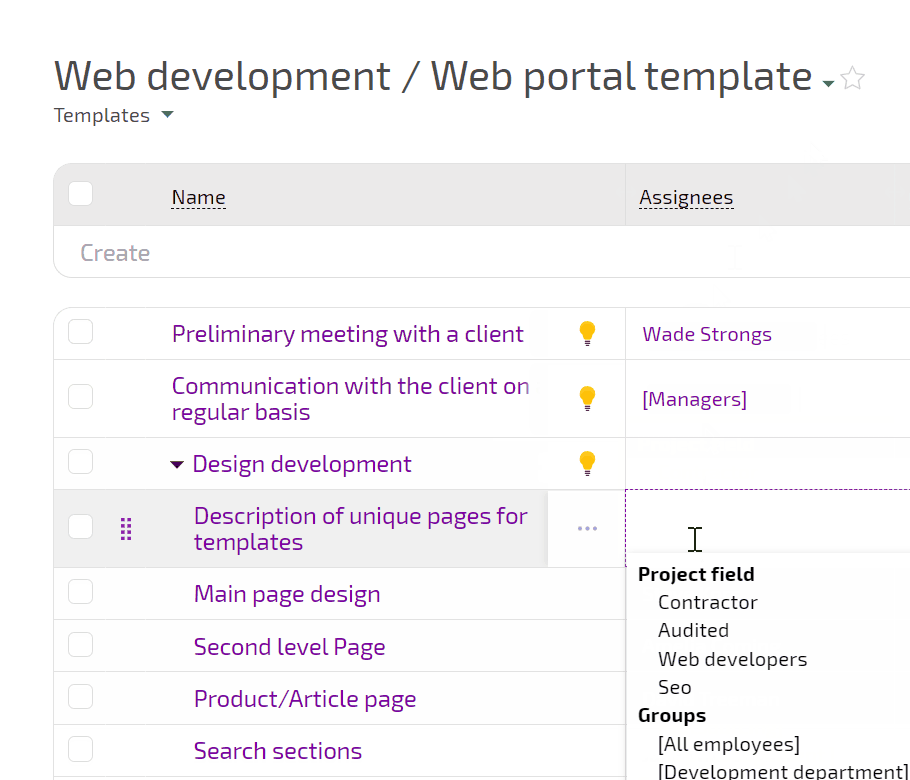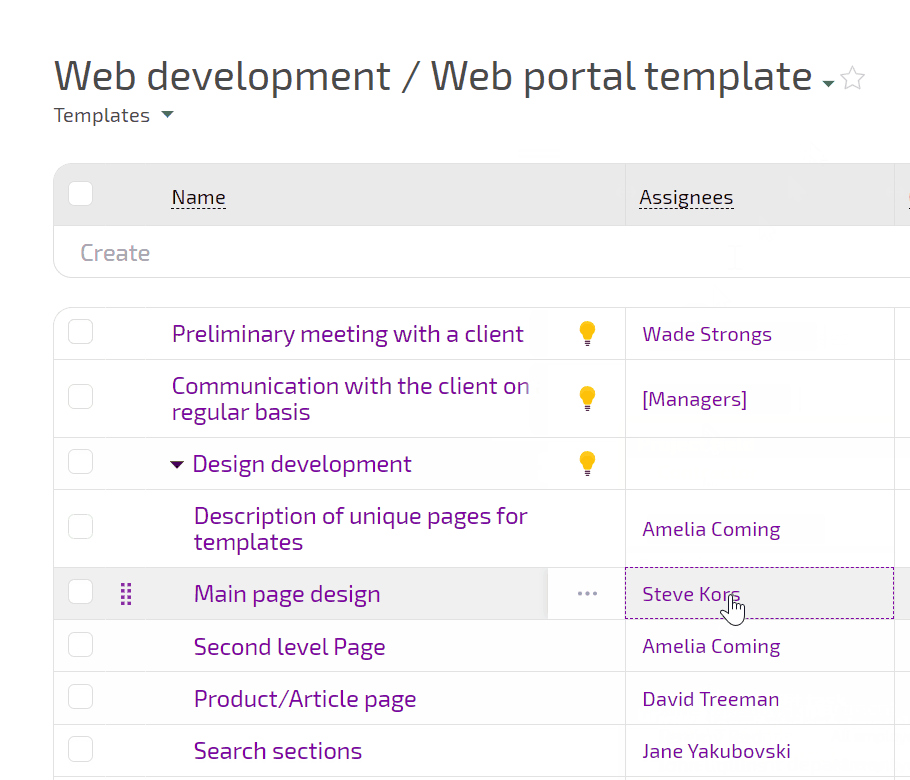
Planfix connects intake, experiment planning, execution, incidents, and decisions in one flow. You see how requests turn into experiments, which runs were affected by incidents, and what actions closed each investigation.
Your R&D team works in a single reliable system. Requests are clear, experiments traceable, and incidents handled with discipline. You move from past experiments to current status and next steps in a few clicks instead of late night searches.
Connect communication, storage, automation, and mapping tools so R&D data flows into one auditable system.